Ultra-Fast Polymerization
The proprietary cyanoacrylate formulation achieves complete solidification in less than one second upon contact with blood, ensuring precise vein occlusion with minimal risk of distal embolization.
Precision engineering meets medical innovation in a revolutionary non-thermal varicose vein treatment system.
VenaBlock® represents a paradigm shift in varicose vein treatment. Unlike thermal ablation methods that require tumescent anesthesia and carry risks of nerve damage, VenaBlock® uses a proprietary cyanoacrylate adhesive that polymerizes in under one second, permanently sealing incompetent veins without heat.
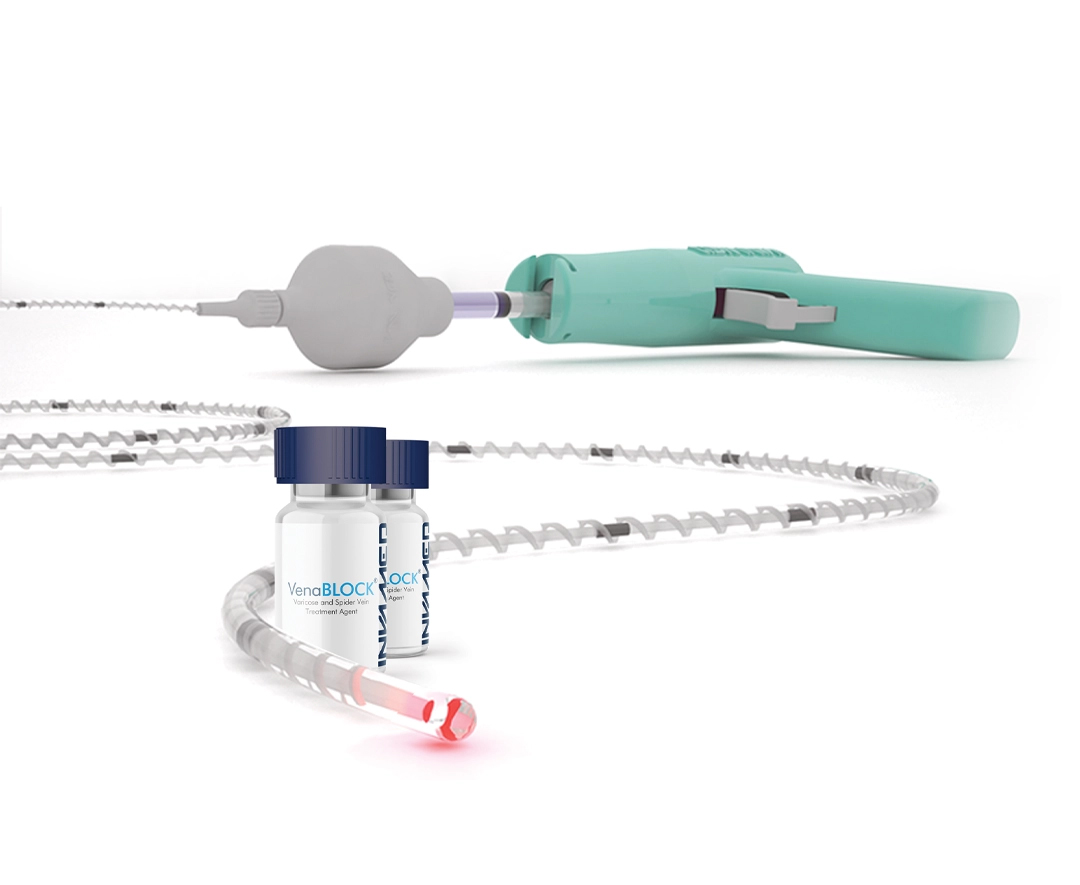
The proprietary cyanoacrylate formulation achieves complete solidification in less than one second upon contact with blood, ensuring precise vein occlusion with minimal risk of distal embolization.
The integrated red laser beam projects through the catheter tip, providing enhanced visibility under ultrasound imaging and enabling accurate positioning even in tortuous venous segments.
High kink-resistance Teflon® braided construction allows smooth navigation through complex venous anatomy without buckling, available in 4F, 5F, and 6F sizes for optimal vessel matching.
Zero thermal energy eliminates the risk of nerve injury, skin burns, and post-procedural pain commonly associated with laser and radiofrequency ablation techniques.
Contact our medical affairs team for detailed product specifications, IFU documentation, and clinical support.